Research
Improving Sweet Corn
The Settles lab group is part of a multi-institutional team, led by Dr. Marcio Resende at UF, awarded a $7.3 million-dollar grant funded by the National Institute of Food and Agriculture involving laboratories from the University of Florida, University of Wisconsin, Washington State University, and Iowa State University focused on improving sweet corn. The study is multi-disciplinary and includes projects focused on various aspects of sweet corn improvement including identifying genetic traits related to taste, shelf life, and germination.
American eating habits are changing, including a decrease in vegetable consumption. While sweet corn is one of the most popularly consumed vegetables, sweet corn has a narrow range of genetic diversity. This is a major issue when considering increases in pest and abiotic stresses on crops due to climate change. Therefore, identifying genetic traits responsible for the success of sweet corn is imperative to protecting this favored vegetable as environments continue to change due to global warming.
Goals of this multi-faceted project include:
-
Developing new breeding technologies to address seed company and processor stakeholder’s concerns regarding limitations in sweet corn production
-
Investigating genetic mechanisms in sweet corn required for harvest and marketability, insect and disease resistance, cold tolerance, and eating quality and aroma
-
Determining physiological and economic trade-offs for consumer preference and valuation of traits

Machine Vision
Our lab is interested in how biochemical and genetic changes in plant seeds impact the seed’s growth and development, nutritional quality, and performance when planted in a field. We have developed several ‘machine vision’ platforms that rapidly collect data on seeds and the seedlings they produce. These platforms allow us to phenotype large populations of seeds to uncover genes that affect the seeds quality and performance.
Single Kernel Near-Infrared Spectroscopy
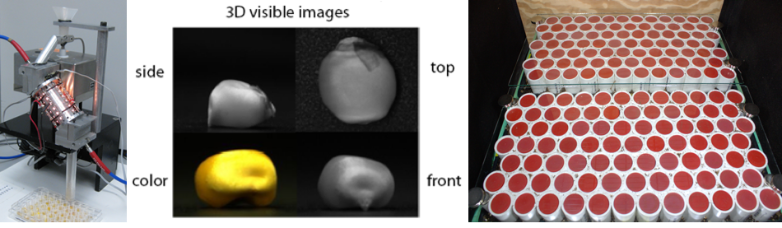
Near infrared spectroscopy (NIRS) is a ‘machine vision’ tool that can rapidly and non-destructively estimate the chemical composition of biological materials. Specific regions of the near infrared spectrum of light (700 nm to 2500 nm) are absorbed by the vibrations of chemical bonds within the biological material. The pattern of NIR absorption can be used to estimate the amount of a particular constituent. NIRS is fast (taking seconds to provide an estimate), non-destructive (whole samples are used), and versatile (able to estimate multiple chemicals at once). We have developed a NIRS platform that is designed for high-throughput phenotyping of individual seeds. The platform collects precise weight and a NIR spectral profile of an individual seed in 4 seconds. We have developed mathematical models for translating the NIR spectra into predictions of numerous seed traits including the amount of starch, protein, oil in the seed as well as seed density, volume, air space, weight, and seed coat (pericarp) thickness. We can phenotype corn, soybean(1,2), common bean, and peas. The goal is to uncover the genes that affect seed composition, size, shape, and potentially field performance.
VIGOR: A machine-vision seedling emergence assay
Trillions of seeds are planted in the US annually with the expectation that the seed will germinate and emerge from the soil as a robust seedling. However, a challenging soil bed environment can delay or even prevent emergence resulting in reduced yield. We have designed an in lab-based assay that allows us to approximate a field environment to mine natural diversity for genes that can improve seedling emergence under challenging conditions. VIGOR is a ‘machine vision’ tool for monitoring seedling emergence over time in a controlled environment. Seeds are planted into individual cells which allows tracking of emergence time on a single seed basis. Fixed position cameras monitor 168 cells by capturing images every 30 minutes over the course of the experiment. Image stacks are uploaded to the CyVerse cyberinfrastructure for image processing and extraction of emergence time. The image below on the right shows a single image containing 168 cells. The red caps act as both a signal amplifier for an emerging seedling and as a cap to keep the seedling from occluding the view of distal emerging seedlings. We are using the VIGOR assay to map genes that improve seedling emergence time and percentage under cold conditions. We are also combining the single kernel information obtained from the skNIR with the emergence profiles to explore the connections between the seed traits and seedling emergence under cold soil conditions.
Genetics of Seed Development
Our lab has identified two rough (rgh) mutants, rough endosperm 3 (rgh3) and rna binding motif 48 (rbm48), that encode RNA splicing factors and cause defects in endosperm development when mutated. RNA-seq data analysis found that U12 splicing is defective in both rgh3 and rbm48 mutants. U12-type introns need to be efficiently spliced for endosperm cells to switch from proliferation to differentiation in early seed development. Our analysis of rgh3 and rbm48 unveiled deep conservation of U12 RNA splicing factors between animals and plants. Further analysis across biological kingdoms revealed that RGH3 and RBM48 are co-selected throughout eukaryotes. We used this cross-kingdom conservation to identify maize armadillo-repeat containg7 (ARMC7) as an RBM48 interacting protein, and novel RNA Recognition Motif (RRM) domains that display co-selection with these previously undiscovered U12 splicing factors. In the lab, we continue to work towards linking these novel U12 splicing mutants to specific seed developmental defects.
Our work also demonstrated direct protein-protein interaction of U12 splicing factors RBM48 and RGH3 with both U2AF1 and U2AF2, subunits of core U2 splicing factor U2AF. This indicates an interaction between major and minor spliceosomes during splice site selection, which may explain observations of defective splicing of U2 introns that flank U12 introns in rgh3 and rbm48 mutants. We will continue to explore the role of plant specific splicing factors on RNA processing during seed development and their targets.
Additionally, our lab is interested in parent-of-origin effects during maize seed development. We have screened a large number of rgh mutants in maize looking for parent-of-origin mutants. We have identified six maternal rgh endosperm (mre) mutants, and three paternal rgh endosperm (pre) mutants. We found that many maize parent-of-origin effects display incomplete penetrance of kernel phenotypes.

Algae in Space
Most recently, our lab has become interested in studying the growth of algae in space. Algae are an excellent option for use in bioregenerative life support systems as humans continue to explore the idea of life in space. Expansion of the human population to the Moon or Mars is getting closer to becoming a reality. Therefore, it is critical to determine methods of continuously producing food in space, as opposed to having food delivered like what is currently the norm for astronaut life on the International Space Station. Bioregenerative life support systems are one proposed solution for this problem which feature the use of photosynthetic organisms to produce oxygen, eliminate CO2, and serve as food sources. Due to their small size and nutritional value, algae are an ideal choice for bioregenerative life support systems.
In 2018, our lab flew an experiment to the International Space Station in partnership with NASA to study the growth and mutation load of a common algal species, Chlamydomanas reinhardtii (commonly termed Chlamy), in space named Space Algae. This experiment involved growing Chlamy in liquid culture in tissue culture bags for several passages, and sampling cells from each passage for DNA extraction and sequencing. We found that the algae experienced an increased mutation load in spaceflight, and a unique mutation signature not seen before on Earth.
Spaceflight is a very complex set of stressors, including primarily microgravity and ionizing radiation. The experimental design of our original space algae experiment limited our ability to determine what the exact stress was in spaceflight that triggered the increased mutation load in Chlamy. Recently, however, our lab was awarded two different grants to better dissect spaceflight stress! Over the next few years, we will be analyzing the effects of chronic exposure to ionizing radiation in both Chlamy and a popular cyanobacteria found in health supplements, Spirulina, here on Earth. Spirulina (Arthospira platensis) is known to be more resistant to acute ionizing radiation compared to Chlamy, so we anticipate a lower mutation load than what we observed in Chlamy. We will also be sending both Chlamy and Spirulina to the International Space Station to be grown and passaged for several generations in spaceflight. We hope that these projects will be help determine if Spirulina is an ideal candidate for spaceflight bioregenerative life support systems.

